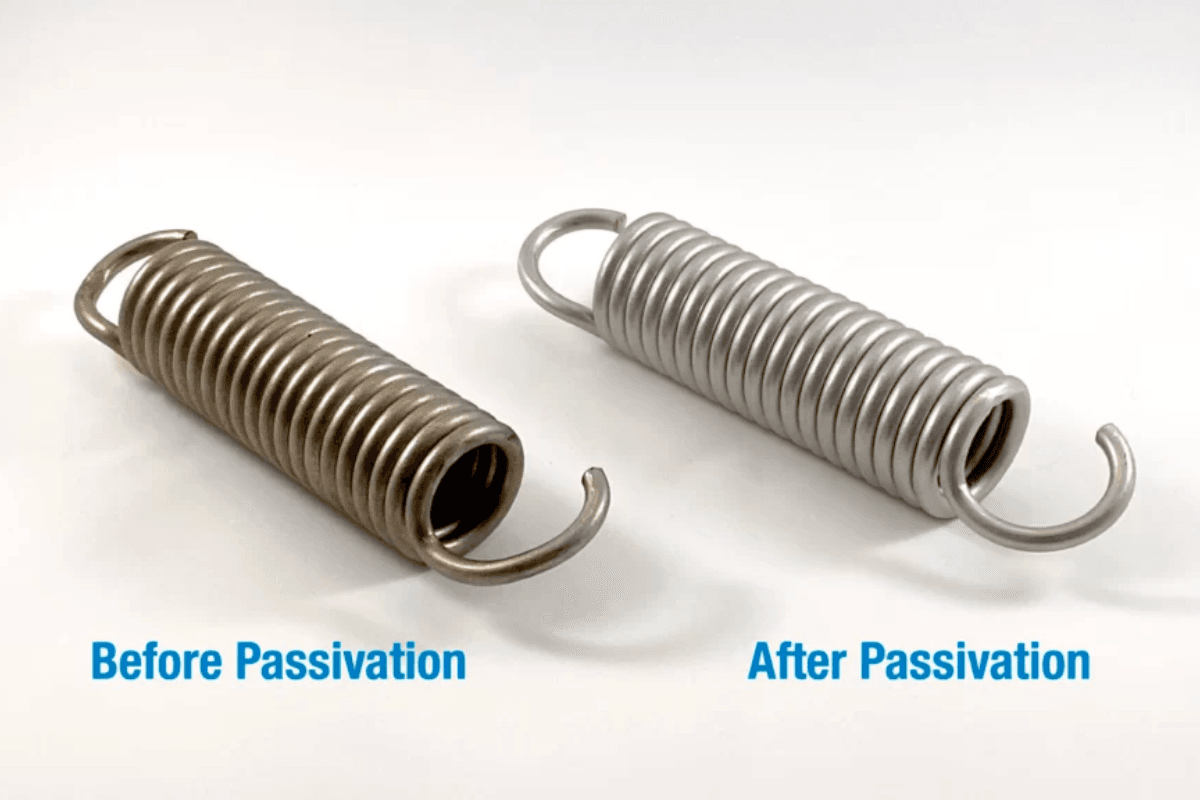
Pasivación Es un proceso de tratamiento químico crítico utilizado principalmente para mejorar la resistencia a la corrosión de los metales, particularmente el acero inoxidable.
Este proceso implica la creación de una capa protectora de óxido en la superficie del metal, lo que reduce significativamente su reactividad con los elementos ambientales.

Definición y mecanismo
Este método implica tratar una superficie metálica con ácido para eliminar contaminantes, permitiendo la restauración de una fina capa protectora de óxido.
Esta capa de óxido actúa como una barrera, evitando que el metal reaccione con la humedad y el oxígeno, que son factores clave que contribuyen a la corrosión.
¿Qué es la pasivación?
La pasivación es un proceso químico que se utiliza para eliminar el hierro libre y otros contaminantes de la superficie de los metales, especialmente del acero inoxidable. Durante la fabricación, las piezas metálicas están expuestas a elementos como grasa, aceites y partículas de hierro, que pueden provocar contaminación de la superficie. El proceso de pasivación limpia el metal y crea una capa protectora de óxido que mejora la resistencia a la corrosión.
Cómo funciona la pasivación
Este tratamiento generalmente implica sumergir el metal en una solución ácida, como ácido nítrico o cítrico, para eliminar los contaminantes de la superficie. A continuación, se muestra un desglose simplificado de los pasos involucrados en la pasivación:
- Limpieza: La superficie metálica se limpia a fondo para eliminar aceites, suciedad y otros contaminantes de la superficie.
- Ácido Baño: El metal limpio se sumerge en un baño ácido, que elimina las partículas de hierro libres y otras impurezas metálicas.
- Enjuague: Después del baño ácido, el metal se enjuaga con agua para eliminar los ácidos y contaminantes restantes.
- El secado: El paso final implica secar el metal para evitar manchas de agua o mayor contaminación.
Este proceso no solo limpia el metal, sino que también promueve la formación de una fina capa de óxido transparente. Esta capa de óxido protege la superficie del metal de la reacción con el medio ambiente, lo que reduce el riesgo de corrosión.
Cabe señalar que la pasivación es diferente del decapado. El decapado elimina principalmente la película de óxido y otros depósitos de la superficie del metal mediante un ácido fuerte, mientras que este método mejora la resistencia a la corrosión de los metales mediante la formación de una nueva capa protectora.
Tipos de pasivación
- Pasivación química:Este es el tipo más común de pasivación, que implica sumergir el metal en una solución química para eliminar impurezas y contaminantes.
- Pasivación electroquímica:Este tipo de pasivación implica la aplicación de una corriente eléctrica al metal para crear una capa protectora.
- Pasivación mecánica:Este tipo de pasivación implica el uso de medios mecánicos, como el chorro de arena o el pulido, para eliminar impurezas y contaminantes de la superficie del metal.
¿Por qué es importante la pasivación?
- Resistencia a la corrosión mejorada: Uno de los principales beneficios de la pasivación es la mejora de la resistencia a la corrosión. Al eliminar los contaminantes y formar una capa de óxido, la pasivación ayuda a los metales a resistir la oxidación y el deterioro en entornos hostiles.
- Mayor vida útil de los componentes: Los metales pasivados duran más, especialmente cuando se exponen a la humedad, a productos químicos o a condiciones extremas. Esto garantiza que los equipos y los componentes mantengan su integridad y su funcionamiento a lo largo del tiempo.
- Estética y acabado superficial mejorados: Este proceso también mejora el atractivo estético del metal al proporcionar un acabado limpio y uniforme. Esto es especialmente importante en industrias como la aeroespacial, la de equipos médicos y la de procesamiento de alimentos, donde tanto el rendimiento como la apariencia son fundamentales.
- Cumplimiento de los estándares de la industria: Muchas industrias, incluidas la aeroespacial, la automotriz y la médica, tienen estándares estrictos de resistencia a la corrosión y limpieza de metales. La pasivación ayuda a los fabricantes a cumplir con estos requisitos al garantizar que las superficies metálicas estén libres de contaminantes.
¿Cuáles son los principales beneficios de la pasivación del acero inoxidable?
La pasivación del acero inoxidable ofrece varias ventajas importantes que mejoran su rendimiento y longevidad. Estas son las principales:
- Mayor resistencia a la corrosión: El principal beneficio de la pasivación es la mejora de la resistencia a la corrosión. Al eliminar el hierro libre y otros contaminantes de la superficie, la pasivación ayuda a restaurar y espesar la capa protectora de óxido de cromo, lo que hace que el acero inoxidable sea más resistente al óxido y a la degradación ambiental. Esto es particularmente crucial para los componentes de acero inoxidable expuestos a la humedad y al aire, como los que se utilizan en aplicaciones aeroespaciales y médicas.
- Mayor vida útil de los componentes: La pasivación no solo mejora la resistencia a la corrosión, sino que también extiende la vida útil de las piezas de acero inoxidable. Al mantener la integridad de la capa protectora, la pasivación reduce la probabilidad de formación de óxido y degradación, lo que puede generar reemplazos costosos y tiempos de inactividad en entornos industriales.
- Apariencia estética mejorada: La pasivación también puede restaurar el atractivo visual de las superficies de acero inoxidable. Elimina la decoloración y las imperfecciones causadas por la oxidación y los contaminantes de la superficie, lo que da como resultado un acabado brillante y limpio que suele ser deseable en productos de consumo y aplicaciones arquitectónicas.
- Rentabilidad y reducción del tiempo de inactividad: Los tratamientos de pasivación periódicos pueden reducir las paradas de mantenimiento y las reparaciones, lo que, en última instancia, permite ahorrar dinero. Al evitar la corrosión y mantener la integridad de los componentes de acero inoxidable, se minimiza la necesidad de realizar procesos de limpieza y restauración exhaustivos, lo que aumenta la eficiencia operativa.
Materiales comunes para pasivación
Si bien el acero inoxidable es el material más comúnmente tratado, la pasivación también se puede aplicar a:
- Aluminio: A menudo tratado mediante anodizado, que forma una capa protectora de óxido.
- Titanio y magnesio: también se benefician de tratamientos similares para mejorar su resistencia a la corrosión.
Aplicaciones de la pasivación
La pasivación se utiliza en una amplia variedad de industrias en las que la resistencia a la corrosión y la limpieza son importantes. Algunas aplicaciones comunes incluyen:
- Componentes aeroespaciales: Las piezas metálicas utilizadas en la industria aeroespacial requieren altos niveles de resistencia a la corrosión para garantizar la seguridad y la longevidad de las aeronaves.
- Dispositivos médicos: Los instrumentos e implantes de acero inoxidable en el campo médico deben pasivarse para evitar la corrosión y la contaminación en entornos estériles.
- Piezas de automoción: La pasivación ayuda a proteger componentes críticos, como sujetadores y piezas del motor, del óxido y el daño ambiental.
- Equipos de procesamiento de alimentos: En la industria alimentaria, las superficies de acero inoxidable a menudo se pasivan para evitar la contaminación y mantener la higiene.
Conclusión
En resumen, la pasivación es un proceso esencial en la ingeniería de materiales, en particular para metales expuestos a entornos hostiles. Al restaurar y mejorar eficazmente la capa protectora de óxido, la pasivación garantiza la longevidad y la confiabilidad de los componentes metálicos en diversas aplicaciones, desde la construcción hasta la microelectrónica.
Obtenga más información sobre las diferencias entre Tratamientos mecánicos y químicos de superficies en nuestro Técnicas de acabado de superficies página.
Preguntas frecuentes
P: ¿En qué se diferencia la pasivación del decapado?
A: Si bien ambos procesos limpian la superficie del metal, el decapado elimina incrustaciones, óxidos e impurezas utilizando ácidos más fuertes, mientras que la pasivación se centra en mejorar la resistencia a la corrosión formando una capa protectora de óxido.
P: ¿Qué metales se pasivan habitualmente?
A: El acero inoxidable es el material más común para la pasivación, pero el aluminio, el titanio y el magnesio también pueden sufrir pasivación para mejorar su resistencia a la corrosión.
P: ¿Qué industrias se benefician más del proceso de pasivación?
A: Industrias como la aeroespacial, la de dispositivos médicos, la automotriz y la de procesamiento de alimentos se benefician de la pasivación debido a la necesidad de una alta resistencia a la corrosión y limpieza en los componentes metálicos.
P: ¿Qué es la pasivación y por qué es importante para el tratamiento de metales?
A: La pasivación es un proceso químico que se utiliza para mejorar la resistencia a la corrosión de los metales, en particular del acero inoxidable. Elimina las impurezas y forma una capa de óxido protectora, aumentando la durabilidad y la vida útil de los componentes metálicos.